The Haber process is a method of Ammonia production developed by Fritz Haber. This is the most common way of producing ammonia, and usually occurs at an industrial level. The Haber process mixes nitrogen and oxygen to produce ammonia through an exothermic reaction:
N2 (g) + 3H2(g) ↔ 2NH3 (g) Δ=-92kJ
(N2 (g) + 3H2(g) ↔ 2NH3 (g) +Heat)
Pressure is kept at 200kPa. There is 4 moles of substance on the left (one mole of nitrogen and three moles of hydrogen) and 2 moles on the right (two moles of ammonia).The equilibrium to shift to the right (to the side with fewer moles) as the pressure is increased, in doing so, more ammonia is produced.
Temperature is maintained at 400-450oC. The equation suggests that heat should be minimized to maximize ammonia production, however this slows down the reaction and produces very little ammonia. High temperatures speed the reaction up so that even though the equilibrium shifts left, the amount of ammonia produced is still higher than when temperatures are reduced and the reaction slows down. At this temperature, Ammonia production is maximized at a 15% yield.
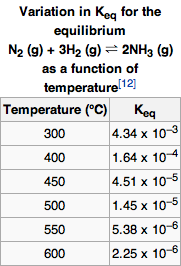
The table shows the relationship between the equilibriumconstant and the temperature.A large equilibrium concentration indicates large amounts of products, and in this case, ammonia. Even though the equilibrium constant is largest at 300K, the reaction would occur too slowly for any significant concentrations of ammonia to be produced in a short period of time, making it costly. However, at 400K, the reaction occurs fast enough to maximize the yield of ammonia.
Increasing the Concentration of N2 and H2. To increase the concentrations of NH3, one method is to increase the concentration on the left (i.e. Increase nitrogen and hydrogen concentrations), so that the equilibrium shifts to the right, and more NH3 is produced to adjust this disturbance.
An Iron Catalyst is used to speed the reaction up, it has no effect on the concentrations, only the speed of the reaction increases so that ammonia can be produced more quickly. This reduces the cost of production as it minimizes the time needed to produce ammonia. The iron catalyst also eliminates the need for excessively high temperatures.
Ammonia is the world’s sixth most produced chemical, with 80 Billion kilograms produced annually. Ammonia is primarily used in artificial fertilizers, cleaning products such as detergents and shampoos, and in explosive production. It is a key ingredient in the agricultural sector (83% of ammonia produced is used in the agricultural sector) as it is used in artificial fertilizers that significantly increases crop yield, which is essential to meet the demands of the growing population. There are however negative impacts, such as increased soil acidity, which can be harmful to bacteria and small insects that live on the land covered in fertilizers. In addition, the fertilizer can leach into drains and water bodies, which can increase the toxicity of the water and lead to rapid algae growth on these bodies of water, which can kill organisms within the pond as light is blocked. The chemicals in the cleaning products can also leach into water sources, causing them to become more toxic and hazardous. Water that contains shampoo can increase the toxicity of the water which it drains into.
Work Cited
1. Fahs, B. (n.d.). Are Synthetic Fertilizers Bad? | eHow.com. eHow | How to Videos, Articles & More – Discover the expert in you. | eHow.com. Retrieved May 4, 2013, from http://www.ehow.com/facts_7248424_synthetic-fertilizers-bad_.html
2. Ammonia fertilizer and soil pH. (n.d.). Iowa State University Extension and Outreach. Retrieved May 4, 2013, from http://www.extension.iastate.edu/nwcrops/fertilizer_and_soil_ph.htm
3. BBC – GCSE Bitesize: Ammonia and the Haber process. (n.d.). BBC – Homepage. Retrieved May 4, 2013, from http://www.bbc.co.uk/schools/gcsebitesize
4. Kessel, H. (2003). Nelson chemistry 12. Toronto: Thomson Nelson.
5. Zmaczynski – Haber Process. (n.d.). Princeton University – Home. Retrieved May 5, 2013, from http://www.princeton.edu/~hos/mike/texts/readmach/zmaczynski.htm
6. Mullin, S. (n.d.). The Effects of Inorganic Fertilizers | eHow.com. eHow | How to Videos, Articles & More – Discover the expert in you. | eHow.com. Retrieved May 7, 2013, from http://www.ehow.com/list_7302511_effects-inorganic-fertilizers.html



Will the speed of the reaction continue to increase when the temperature increases or is their a limit for the speed of the process?
Hey Jabril, thank you for your question. If the temperature is continued to be increased, the speed of the reaction does increase as there is a greater input of kinetic energy. The molecules within the reaction collide at a faster rate per unit time, and therefore the reaction occurs faster. There is a limit as some of the chemical species are altered and the reaction begins to slow down and stop, however in terms of the Haber process, it is more important to look at the optimum temperature (which is between 400oC and 450oC). Even though the speed of the reaction increases, the reaction is exothermic, meaning that as the speed of the reaction increases, the equilibrium shifts left at the same time, away from the ammonia. Cooling the system will cause the equilibrium to shift right, however will occur to slowly for the reaction to produce significant amounts of ammonia with a fixed time frame. Therefore there has to be an optimum temperature at which the most of ammonia is produced.
For further reading, this site expands on what is mentioned above: http://www.chemguide.co.uk/physical/equilibria/haber.html
For further reading, this site expands on the factors that affect the rates of chemical reaction (refer to the temperature section): http://chemistry.about.com/od/stoichiometry/a/reactionrate.htm
Hello Abhishek 🙂 Ariful here 😀
Could you expand a bit on the iron catalyst? I am not sure what it is 😀
Hey Ariful, thanks for your comment. We learnt that the catalyst has no effect on the equilibrium of the system, but speeds up both the forward and backward reaction. Without the iron catalyst, the reaction would occur far to slowly for any significant amounts of Ammonia to be produced. The iron catalyst allows for the same percentage of ammonia to be produced in a far shorter amount of time. I say percentage because even if the iron catalyst was not present there will still be a 15% yield, the iron catalyst just allows that 15% of ammonia to be produced much faster. The reason it is used is mostly due to cost efficiency, the more ammonia that is produced, the greater the profits. The less time these machines take to produced a fixed amount of ammonia, their costs will be.
To maximize the yield of ammonia, you mentioned that there has to be a compromise between a high and low temperature such that there is a balance between the shift in the reaction to the right (caused by a lower temperature) and the higher rate of reaction resulting from a higher temperature,
Does a similar relationship exist when the reaction occurs in higher pressure, as opposed to a lower one?
Hey Raman, thank you for your question. No, the pressure does not affect the speed of the reaction, it only affects the equilibrium. Therefore, manufacturers of ammonia try to keep the maximum possible pressure. The reason that the pressure is usually never increased above 200kPa is because the higher the pressure, the most costly it is! Specialized (expensive) pipes and tanks must be used to withstand the enormous pressures required to go beyond 200 kPa, and as a result, the pressure is usually maintained at 200kPa. The video that accompanies the blog explains why the pressure is maintained at 200 kPa, please check it out if you are interested.