Important Background Information:
Acids: an acid is a chemical species that can donate a proton (H+) [1].
Bases: a base is a chemical species that can accept a proton [1].
- The pH of a solution is measured through the concentration of H+ ions in that solution.
- Acid-base buffers: a solution that resists a change in pH of a solution when hydrogen ions or hydroxide ions are added or removed [1].
- The conjugate base of an acid is the species formed after the acid loses a proton. The base then gains another proton to return to the acid. The acid and its conjugate base exist in equilibrium in a solution [1].
- A buffer consists of a weak acid, and its conjugate base.
- A buffer works because the concentration of the weak acids and its conjugate base are larger than the amount of protons or hydroxide ions that are added or removed [1].
- When protons are added to the solution, some of the base from the buffer is converted to a weak-acid using up most of the protons added [1].
- When hydroxide ions are added to the solution (or protons are removed), protons are dissociated from some of the weak-acid molecules, and convert them to the base of the buffer to compensate for the protons that are removed [1].
- The change in acid and base concentrations is very small relative to the amounts of the H+ or OH– present in solution, therefore the ratio of acid to base changes only slightly and the effect on the pH of the solution is small [1].
Biology Background and Homeostasis:
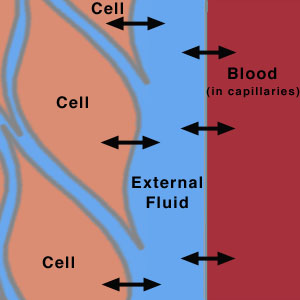
All cells in the body are constantly exchanging chemicals with the external fluid surrounding them [1]. This is the process by which the cell receives nutrients and ions and removes waste products. The external fluid acts as method of exchange between the cell and blood vessels primarily through the process of diffusion through membrane channels through a concentration gradient (osmosis). Since blood is responsible for the maintenance of the cell, the chemical composition of blood is vital to the wellbeing of the cell and therefore tissue and organs. This means that the concentration of ions in blood is always regulated and must be kept relatively constant in order to maintain proper chemical composition inside the cell. This is otherwise known as homeostasis. An example of this is the regulation of pH of the blood. If the pH of the blood is too low, meaning there are too many H+ ions in the blood, this in turn will result in an excess of H+ ions in the cell, and vice versa.
Carbonic-Acid Bicarbonate Buffer System and Le Châtelier’s Principle:
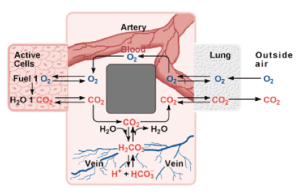
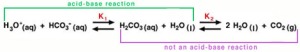
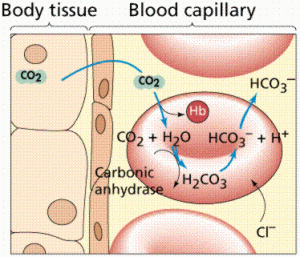
The are several ways in which the body maintains homeostasis in the blood. The most important way of maintaining the pH of the blood is through buffers dissolved in the blood. There are three important buffer systems in our bodies, the carbonic-acid bicarbonate buffer system, the phosphate buffer system, and the protein buffer system [2]. The most important buffer system in the blood in the carbonic-acid bicarbonate system. To understand how the carbonic-acid bicarbonate system works, one must first understand how buffers work. A buffer is essentially a molecule that has the tendency to either release of bind with hydrogen ions to maintain a pH level [2]. A buffer is made up of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is used to resist changes in pH. In the bicarbonate buffer system, carbon dioxide combines with water and forms carbonic acid, which in turn dissociates and forms hydrogen ions and bicarbonate. The carbon dioxide comes from the lungs during exhalation. This system is at equilibrium until it is disturbed by an outside factor, in which case the system must shift either left or right to resist the change. For example, an excess of carbon dioxide in the blood will result in the dissociation of more H+ ions (acidemia) and decrease the pH of the blood. According to Le Châtelier’s Principle, any disturbance of a system at equilibrium is compensated for through a shift in the chemical equilibrium [3]. In this case, some of the hydrogen ions are going to associate with bicarbonate to form carbonic acid, which results in a smaller net increase of acidity.
Importance of maintaining pH of the Blood:
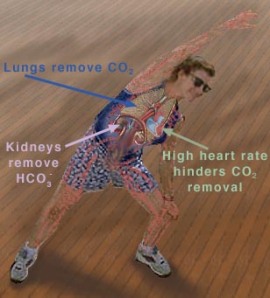
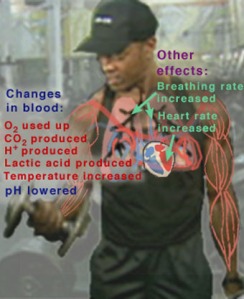
The pH of blood at its normal level is 7.4, and the blood must remain within a narrow range of this pH as drastic changes cannot be tolerated by the cells, and can result in the destruction of cells; this what makes pH regulation so important. A pH above 7.4 results in alkalosis, and anything below 7.4 results in acidosis of the blood. An abnormal pH level of the blood can be due to several factors, overexerting your body through exercise, improper diet, drug consumption, and other biological factors such as kidney failure (since the kidneys are responsible for removing excess H+ ions and other components of the pH buffer [3]). The body can take immediate action to try and counteract the change in pH. For example, during exercise, the muscles use up a lot of oxygen to break down glucose for energy; CO2 and H+ are the byproducts of glycolysis. The production and removal of CO2 and H+ causes chemical changes in the blood, which in turn cause the pH of the blood to drop. If the pH of the body drops below 7.4, a condition known as acidosis results. This can be very serious, because many of the chemical reactions that occur in the body, especially those involving proteins are pH-dependent (increased pH can denature protein) [4]. Ideally, the pH of the blood should be maintained at 7.4. A drop in pH below 6.8 or a rise above 7.8 can result in death.
Acid Base balance:
http://www.youtube.com/watch?v=i_pTaTveCCo
[1] Frey, R., & Casiday, R. (n.d.). pH Buffers in the Blood. Department of Chemistry | Washington University in St. Louis. Retrieved May 4, 2013, from http://www.chemistry.wustl.edu/~edudev/L
[2] Tamarkin, D. A. (n.d.). Buffers. STCC Faculty Webpages. Retrieved May 4, 2013, from http://faculty.stcc.edu/AandP/AP/AP2page
[3] Hasan, A. (2008). Handbook of Blood Gas/Acid–Base Interpretation. Springer: Google eBook.
[4] Gennari, F. J. (2005). Acid-base disorders and their treatment. Boca Raton: Taylor & Francis.
[5] Seldin, D. W. (1989). The Regulation of acid-base balance. New York: Raven Press.
[6] Clark, J. (n.d.). Buffer Solutions. Chemistry Guide. Retrieved May 4, 2013, from http:/http://www.chemguide.co.uk/physical/ac

Awesome post! Though I do have a question on the topic. Does body temperature have any effect on the equilibrium? If someone has a fever would this affect the buffer system in anyway?
How does the glomerulus prevent hydrogen ions from passing through it and being in the glomerular filtrate?