Acid Base Balance and Buffers (Acid & Base pH)
By: Harsaan Nithiananthan
Everyday, though we do not normally recognize it, we are constantly engulfing acids and bases into our body. In other words this is known as the consummation of buffers within the human body. Now you must be wondering what buffers are. That will be explained in the next paragraph.
What are Buffers?
A buffer is known as an aqueous solution that contains a high stable of pH (percentage hydrogen). Stirring large volumes of weak acid or weak base together with its conjugate can make buffers. When we have the hydrogen ions added to any buffer, the base ends up neutralizing due to the properties of the acid. All together, when looking at the pH scale, all of these different mixtures of acids and bases to buffer will not drastically change the value of the pH scale.
Acids and Bases in Reality
Today’s generation’s lifestyle is to eat anything we can get our hands on and what ever pleases or taste for food but in reality our bodies are supposed to consume specific amounts of acids and bases per day. Our body must have an even balance between both acids and bases.
Most people of unaware of what they eat in today’s generation of busy working people but in reality we must understand what we are consuming. This is why the pH scale is of great use. The pH scale was created to provide a specific measure on a scale, which ranges from zero to fourteen of the acidity or even alkalinity of any substance of solution. On the scale seven is known as the neutral point, therefor what ever is lower than seven is acidic and what ever is greater than seven is determined to be a base.
Why is maintaining a balance of Acids and Bases important?
Basically within the human body there is something known as acid base balance, which is the part of human homeostasis in charge of the proper balance of acids, and base that the human body consumes. The acid base balance is the state of equilibrium between the alkalinity and acidity of the body fluids through out the main stream. This can be also named as the hydrogen ion balance since (H+) can be referred to the level of acids and bases. Of course, there would not be a direct effect for an un balanced acid base within the body but for the long run, the symptoms will start to appear. As follows, most substances we eat are acids, which are proton donors while the base substances act as proton acceptors. One example, which is very common, is the orange, which is acidic. But no matter what we eat, our body will some how try to adjust the settings to balance because normally the pH remains commonly constant both outside and inside a cell or many cells.
What are the impact of unbalanced acids and bases?
One very common health effect is chest pain or sometimes known as heart burn. The medication used for these are mainly tums or some sort of substance that would enable the acid to with neutralize with a base or vice versa in order to stop the pain or symptom. From any point if one analyzes, no matter what we eat, there will be no balance of consuming equal amounts of acid and bases but thanks to our machine, known as our own human body, it strives to neutralize and basically bring the acids and base to equilibrium.
Video to learn more -> http://www.youtube.com/watch?v=eeQlkZkCcNY
References
http://www.brighthubeducation.com/science-homework-help/108712-acid-base-balance-in-the-human-body/
http://medical-dictionary.thefreedictionary.com/acid-base+balance

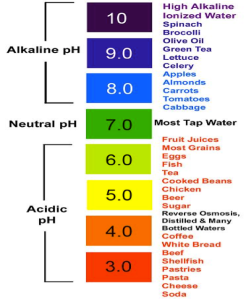
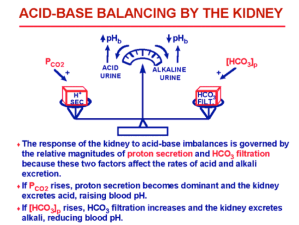
What would be some examples of a buffer, (bicarbonatre system, phosphate system)?
Main two buffer solutions are known as the Acidic Buffer solution which is simply one which has a pH less than 7 and there is an Alkaline buffer solutions which is basically a buffer solution has a pH greater than 7. But to list most of the common buffers there are…
1) Bicarbonate Buffer
2) Phosphate Buffer
3) Protein Buffer
Each buffer has its own specific unique properties.
what is the general equilibrium equation that describes the bicarbonate buffer system and according to le chatelier’s principle which way will the reaction shift when your blood pH is too basic or too acidic?
The main equation that describes the bicarbonate buffer system is known as:
CO2 + H2O = H2CO3 = HCO3 (minus) + H (Plus)
The information is explained in seemas2013’s comment, since both questions were similar, and here is a basic chart that will summarize the shifting pattern.
Predicted Equilibrium Shift
Change in pressure Affects gases only.
Concentration of reactant(s) increased Equilibrium shifts toward products (to the right).
Concentration of product(s) increased Equilibrium shifts toward reactants (to the left).
Temperature increased
(exothermic reaction) Equilibrium shifts toward reactants (to the left).
Temperature increased
(endothermic reaction) Equilibrium shifts toward products (to the right).
As le chatelier’s principle explains, when we have a reactant or product of an equilibrium reaction which we add to any solution that is at equilibrium, the added species will react to change the specific concentrations of the reactants and products in the solution until a new equilibrium is firmly stated.
Well done! what is the equilibrium equation for the buffer system?
When looking at living and breathing things, bicarbonate is very important and actually the Bicarbonate buffer system is the main system for living things, including us humans. This system contains of, carbon dioxide which combines with H20 to give carbonic acid. The carbon dioxide, carbonic acid equilibrium is catalyzed by the enzyme carbonic anhydrous. Then carbonic acid bicarbonate equilibrium is basically proton dissociation/association and needs no catalyst.
The equation would be : CO2 + H2O = H2CO3 = HCO3 (minus) + H (Plus)
How does the equilibrium affect the human body?
Well to rephrase your question I guess you are also asking, how does the buffer affect the human body which leads to your question. The ordinary buffer in a human body is bicarbonate, and its function is to maintain our pH’s at 7.4. The bicarb takes in the extra H+ ions and this will then become carbonic acid, which breaks down to H20 and carbon dioxide, and the CO2 will be exhaled on its next trip to our lungs. For a chemical equation explanation it is shown below.
H+ + HCO3- —> H2CO3 —> H2O + CO2
If there is an excess of base in our bodies then the bicarb is willing to neutralize the base with its hydrogen proton and become a carbonate ion:
base- + HCO3- —> base-H + CO3-2
By slowing down the respiration, more CO2 will collect in the blood stream, and it will mix with H20 to become carbonic acid. When this carbonic acid with 2 H’s sees a carbonate with nothing, they share so they have one each. This results in the body now having 2 bicarbs:
H2CO3 + CO3-2 —-> 2 HCO3-
It is a remarkable system!
You mentioned heartburn as a cause of consuming foods with a high level of acidity, and using tums or a basic type of substance to counteract the change of pH in your body and bring it back to neutral. However, I was wondering what would happen if you were to consume foods with a very alkaline or basic pH, and what kind of measures would you need to take to counteract the change?
That is indeed a good question. Your first question, what would happen if you were to consume foods with a very alkaline or basic pH? Well the answer is, our bodies, for example, the pH of the urine can be alkaline in response to the body being too acidic. The opposite is true as well for basic ph. The body strives all the time to keep the acidity level balanced and base level and in a last effort to protect the cells and tissue the kidneys produce a greater supply of the hormone glutaminase that causes ammonia to be released from the amino acid glutamine. If one was to recognize this, this in fact would be a signal to get on an alkaline diet with vegetables high in sodium because you are too acid. The is really not a specific way of counteracting these scenarios, because our bodies, which we can think as machines were built to counteract these type of situations themselves, with out us even knowing.
Please ignore my first question, my browser was glitching. Your blog is very informative and well planned, i enjoyed reading through it , but i still cant grasp some concept most especially the consequences of not baling basic and acidic meal. In a minimum of 1500 word , can your go over the long term effect of not balancing your acidic and basic meal.
I think you meant to put 150 word summary but thats alright… there are long term affects to having a non balanced meal which means too much of acidity in the body affects all the body’s major systems and is the cause of many diseases. Sometimes it wont be as serious as stated in the previous sentence but it can lead to diarrhea and cause your stomach to feel very painful and empty. Too much of anything isn’t good and the same concept applies here as well. The base gives similar reactions. Having a body which is balanced in acidity and base is able to fluctuate more.
I found you to be very help full but how are you so sure about your facts?