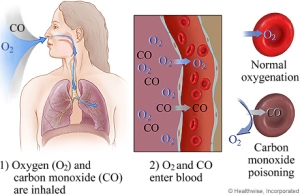
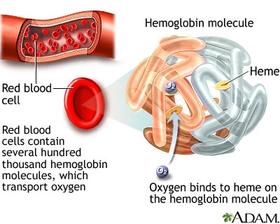
In today’s world there are much chemical equilibrium that tends to be abstract as foregoing section as equilibrium constant, and Le Chatelier’s principle. In addressing the subject of equilibrium, the result can be seen as a process that is involving human health as an importance and maintains the equilibrium in the reaction is between hemoglobin and carbon monoxide. When exposed or inhaled to a harmful gas such as carbon monoxide, “it takes the place of oxygen in hemoglobin, which the red blood pigment carries, oxygen to all parts of the body”.[2][3] Because carbon monoxide binds to hemoglobin which is much stronger than oxygen, this will affect the body causing oxygen starvation throughout the body.
The bonds between the carbon monoxide and the hemoglobin and the equilibrium expression thus becomes: “Hb (aq) + 4CO (g) ⇋ Hb (CO) 4 (aq)”[1] which is called carboxy-hemoglobin. The bonds between hemoglobin and carbon monoxide are approximately 300 times as stronger which indicates that the shift in equilibrium will move towards the right side of the equation of the carboxy-hemoglobin side. In this case K for the hemoglobin-carbon monoxide reaction has a much higher reaction. “The affinity of hemoglobin puts priority on carbon monoxide bonds, which the hemoglobin has bonded to carbon monoxide and is no longer able to carry out oxygen.”[1][2] Carbon monoxide can cause headaches and dizziness, which can be fatal. The reserve effect of carbon monoxide, oxygen has to introduce into the body. It will help with the carboxy- hemoglobin, which will produce oxygenated hemoglobin, along with carbon monoxide:”Hb (CO) 4 (aq) + 4O 2 (g) ⇋ Hb (O 2) 4 (aq) + 4CO (g)”.[1] Carbon monoxide thus produced is dissipated when the person exhales.
[1] http://www.ausetute.com.au/blood.html
[2] http://www.ncbi.nlm.nih.gov/pubmed/3976
[3] http://teachingcases.hematology.org/broudy/erythroprotein.cfm

Why is Carbon Monoxide poisoning and why is it lethal to humans?
Carbon monoxide is very poisonous, when a human inhales carbon monoxide it attaches to the hemoglobin which makes it difficult to release. As you breathe in carbon monoxide it sticks and takes up hemoglobin, and takes up and also binds to the oxygen. Eventually the blood in the body will lose the oxygen which can make you suffocate. To reverse the process is to breath in oxygen at a very high concentration in order to eliminate carbon monoxide.
Your post was very well written and informative. However, I have a few questions. What factors disturb the equilibrium? How does the system reestablish its equilibrium due to the change? What happens when there is an increase or decrease of specific reactants or products? For example, what happens where there is a change in the CO levels?